TIWN
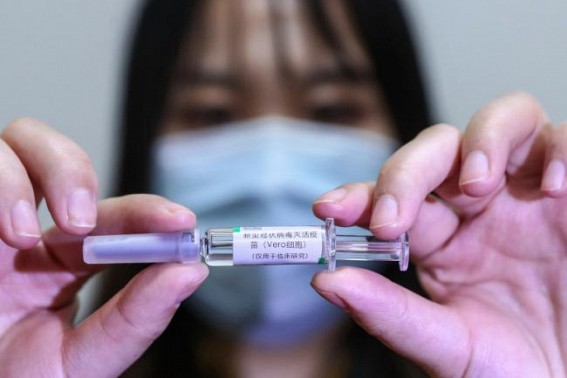
Geneva, May 8 (TIWN) The World Health Organization (WHO) on Friday approved the Covid-19 vaccine developed by China's Sinopharm for emergency use.
The UN health agency signed off on the two-dose vaccine, which is already being deployed in dozens of countries around the world. The WHO has already given emergency use listing to the vaccines being made by Pfizer-BioNTech, Moderna, Johnson and Johnson, and the AstraZeneca jab being produced at separate sites in India and in South Korea. "This afternoon, WHO gave emergency use listing to Sinopharm Beijing's Covid-19 vaccine, making it the sixth vaccine to receive WHO validation for safety, efficacy and quality," WHO chief Tedros Adhanom Ghebreyesus told a news conference. "The Strategic Advisory Group of Experts on Immunisation, or SAGE, has also reviewed the available data, and recommends the vaccine for adults 18 years and older, with a two-dose schedule." An emergency use listing by the WHO paves the way for countries worldwide to quickly approve and import a vaccine for distribution, especially those states without an international-standard regulator of their own. It also opens the door for the jabs to enter the Covax global vaccine-sharing scheme, which aims to provide equitable access to doses around the world and particularly in poorer countries.
- Terror Attack at Jewish Festival left 16 dead, many injured in Australia
- Australian Teens Under 16 Begin Losing Access to Social Media as Nationwide Ban Takes Effect
- Russia launched Massive Missle Attacks in Ukraine
- FIFA Peace Prize Goes To Trump : What are the 8 Wars that Trump Claimed to Stop in 2025?
- Dengue Deaths are Increasing in Bangladesh



